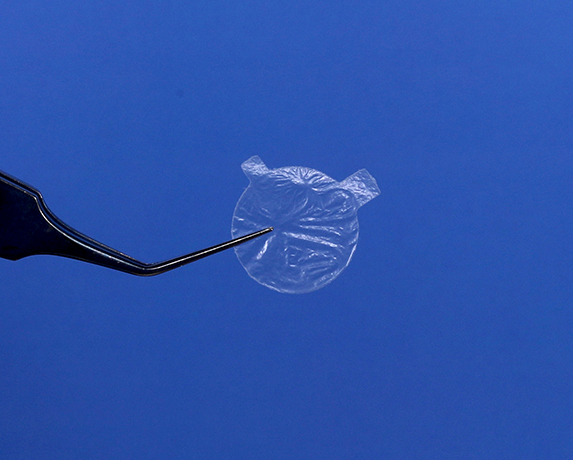
Introducing the new and innovative AmnioTek®-C Processed Human Amniotic Disc representing the latest in regenerative biologics technology for the ocular surface Viable AMT has been successfully employed in the surgical treatment of the ocular surface in well over 100,000 cases over the past decade AmnioTek®-C offers the opthalmic surgeon an effective, natural, minimally processed dehydrated, device-like amniotic membrane tissue featuring anti-inflammatory and anti-adhesive properties ideal for wound covering and wound healing applications. AmnioTek®-C is a proven effective treatment for a host of ocular surface conditions. Safety and Sterility AmnioTek®-C amniotic tissue meets the most stringent donor screening and serological testing standards to ensure safety. AmnioTek®-C is delivered sterile, dry and with a 3 year room-temperature shelf-life greatly reducing storage and shipping logistical costs incurred by other frozen allograft technologies. What is the size of disc? Each disc of AmnioTek-C is 12 mm in diameter, one box of AmnioTek-C has one such piece. What side is the correct orientation of the disc? When the disc is held in such a way that the bigger projection is clock-wise to the smaller one, the epithelial side faces the cornea. What is the shelf life of AmnioTek-C? The shelf life of AmnioTek-C is three years in the box. Once the box is opened it has to be used in the same sterile field. Is it possible to re-sterilize the disc once the box is open? No, the disc cannot be re-sterilized after the packing is open, as sterility is lost once the box is open.