“Introducing the new, innovative addition to the world-wide brand AmnioTek™ Processed dehydrated membrane AmnioTek™-G. This triple thickness 150 microns tissue represents the latest in regenerative biologics technology in supplementing glaucoma surgical applications.”
Safety | Viability | Sterility
Dehydradted AMT has been successfully employed in the surgical treatment of the many ocular surface diseases in well over 1,000,000 cases over the past two decades
Deleted: It is delivered 100% sterile , dry with a 3 year-shelf life and stored at room temperature.This greatly reduces storage issues of refrigeration and shipping logistical cost and questions of sterility by other fresh and frozen AMT technologies.
Structure of Amniotic Membrane
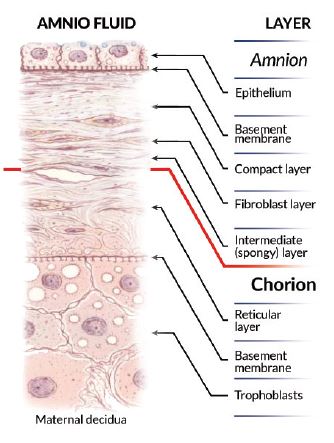
The human amnion is composed of five distinct layers. It contains no blood
vessels or nerves.
The various layer secrete different types of collagen Type IV, V & VII which are present in Extracellular Matrix (ECM). It also has abundant content of specialized proteins such as fibronectin, laminins proteoglycans & glycoproteins which give amniotic membrane is unique healing properties. Also, presence of various growth factors such as Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF), TGF-ß promote healing & growth in the effected area.
Glaucoma Shunt Tube Covering
AmnioTek™-G is the surgical graft for glaucoma specialists because it is transparent, it has high tensile strength and designed thickness. AmnioTek™-G is specifically prepared to cover the three available GDD ( glaucoma drainage devices) tube. AmnioTek™-G can reduce surgical times because it does not require rehydration, it is easy to handle vs the other choices for grafting of tissue banked sclera or pericardium . Product has a 3 years shelf life and doesn’t need any refrigeration or special storage.
In glaucoma drainage device surgery, ophthalmic surgeons need to manage delayed postoperative complications, such as tubal exposure , as this can potentially result in vision-threatening ocular infection.
AmnioTek™-G is semi-transparent and is valuable as a graft for Over-lay of the GDD tube , it is the low immunogenicity, anti-inflammatory, antimicrobial and antifibrotic properties of AmnioTek™-G during embryogenesis that have created increasing interest over the past 15 years.
What is the size of the membrane?
The size of each piece of AmnioTek-G is 2×2 cm, and one box of AmnioTek-G has one such piece.
What is the correct orientation of the graft?
AmnioTek-G is a triple layered graft and it can be put in any orientation as both surfaces are stromal sufaces.
What is the shelf life of AmnioTek-G?
The shelf life of AmnioTek-G is 3 years in the box. Once the box is opened it has to be used in the same sterile field.
Is it possible to re-sterilize the membrane once the box is open?
No, the membrane can not be re-seterilized after the packing is open, as sterility is lost once the box is open.