MOLTENO® Glaucoma Drainage Devices
About
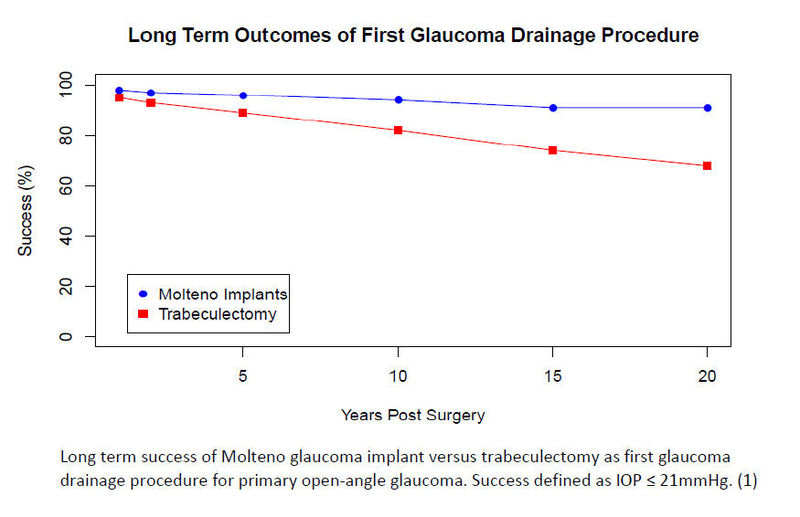
MOLTENO®; Ophthalmic Ltd manufactures the most versatile, safe and effective glaucoma drainage devices with the longest and most comprehensive follow-up of any glaucoma devices on the market (1-6,9-14). Glaucoma drainage devices are also known as glaucoma shunts, drains or implants.
Many leading glaucoma surgeons now prefer the MOLTENO3® S-series implants which feature:
- New anterior suture hole position for fast, easy insertion
- Two sizes (185 and 245mm2), the smaller SS implants are suitable for most cases
- Translimbal, pars plana and ‘piggy-back’ insertion options with one implant
- Can be positioned supra-Tenon’s if necessary
- Best profile for highly myopic eyes with limited space in the orbit
- Plate edge fits snugly between and slightly beneath the adjacent extraocular muscles
- Immediate or delayed drainage (using absorbable suture +/- relieving slit (16))
- Pressure ridge reduces the risk of hypotony
- Pressure ridge and plate design give a permeable bleb with excellent long term control of intraocular pressure (5,6,14)
- MRI safe.
- MOLTENO3® glaucoma implants are also available in the standard profile G-series.
The MOLTENO3® implant range is usually used with an absorbable suture ligature (Vicryl® tie, (13)) to delay drainage, except when treating urgent cases of acutely raised intraocular pressure, neovascular glaucoma, silicone oil induced glaucoma and a few cases where inflammatory exudate or blood is present in the eye.
MOLTENO® Ophthalmic also produce the original Single Plate (S1), the Double Plate range and the Paediatric / Microphthalmic (P1) implants to meet worldwide demand for these Molteno glaucoma drainage devices.
Medication
Hypotensive medication before and after MOLTENO® Implant surgery:
Before surgery: control the intraocular pressure (IOP) optimally to minimise operative complications.
From surgery until the onset of drainage: maintain the IOP in the mid-range, around 15mmHg is ideal in most cases.
Once drainage commences, and in all cases with immediate drainage: Control inflammation, if present, and allow the IOP to rise above 15mmHg (above the episcleral venous pressure). This triggers the process which leads to a more permeable bleb capsule and long-term control of IOP.
From 3 months postoperatively: aim to keep the IOP in the low-normal range (8-12mmHg). When the IOP is well controlled in the months and years after Molteno Implant surgery, using hypotensive agents if necessary, there is a tendency for increasing permeability of the bleb lining to occur over the long term, reducing the IOP further and decreasing the need for hypotensive medication.
CAUTION: Up to 3 months after Molteno implant surgery, miotics, prostaglandin analogues and other topical vasodilating agents should be used with caution as they may increase the episcleral venous pressure and thus increase fibrous tissue deposition and the likelihood of bleb failure.
Single Plate Implant
MOLTENO3® S-series Single Plate Implant (SS & SL)
Recently launched in response to feedback from leading surgeons:
- Easy access to anterior suture holes for fast and easy suturing
- Refined contour and edge details
- Ultra-low profile 0.95mm plate thickness and lower profile pressure ridge
- Single plate simplicity with double plate performance (2)
- Single quadrant surgery with a low profile implant that slips easily into place between and slightly beneath the adjacent extraocular muscles
- Suited to cases with large eyes and little space in the orbit
- Flexible and shaped to fit the eyeball
- Pressure ridge reduces the risk of post-operative hypotony: restricts initial aqueous drainage to the small primary drainage area until the IOP rises sufficiently to lift the tissues and allow drainage of aqueous over the entire plate
- Self-cleaning patented ‘biological valve’ resists blockage by inflammatory exudate, blood clot or fibroblast ingrowth, unlike a mechanical valve (8)
- MRI safe
The pressure ridge on the MOLTENO3® S-series implants has been lowered and rounded to reduce the risk of erosion when the implant is placed supra-Tenon’s (7) or when the Tenon’s tissue and conjunctiva are compromised or poor healing may be expected.
MOLTENO3® G-Series Single Plate Implant (GS & GL)
- Single plate simplicity with double plate performance (2)
- Single quadrant surgery
- Low profile plate that slips easily into place between and slightly beneath the adjacent extraocular muscles
- More flexible and shaped to fit the curvature of the eyeball
- Self-cleaning ‘biological valve’ resists blockage, unlike a mechanic valve (8)
- Excellent long-term control of IOP (2)
- Pressure ridge reduces post-operative hypotony by acting as a ‘biological valve’
- The pressure ridge restricts initial aqueous drainage to the small primary drainage area until the IOP rises sufficiently to lift the tissues and allow drainage of aqueous over the entire plate
- MRI safe
MOLTENO® Single Plate Implant (S1), the original MOLTENO® glaucoma implant
In cost-critical healthcare settings, the original MOLTENO® S1 Implant, particularly when combined with delayed drainage using an absorbable suture such as Vicryl®, gives very satisfactory results. Most surgeons now prefer the Molteno3® range of implants.
The original MOLTENO® S1 Implant is suitable for elderly patients with reduced ciliary body function and for combined cataract and glaucoma operations.
The original MOLTENO® Single Plate Implant (S1) may also be used to provide additional drainage to an already implanted glaucoma device in the ‘piggy-back’ procedure (19).
MRI safe.
MOLTENO® Pressure Ridge Single Plate Implant (D1)
The subsidiary ridge on the MOLTENO® Pressure Ridge Single Plate Implant (D1), when covered by Tenon’s capsule, acts as a pressure-sensitive biological valve which limits the escape of aqueous from the eye and reduces postoperative hypotony.
The Molteno Pressure Ridge Single Plate Implant is suitable for patients with neovascular glaucoma, acute glaucoma in patients with reduced ciliary body function, elderly patients and patients who require a combined cataract and glaucoma procedure (4). Most surgeons now prefer the MOLTENO3® Implants.
The Molteno Pressure Ridge Single Plate Implant is also an option when adding drainage area to a previously inserted implant in the ‘piggy-back’ procedure.
MRI safe.
Double Plate Implant
MOLTENO® Double Plate Implant (L2 & R2)
MOLTENO® Double (twin) Plate Implants provide twice the drainage area of an original MOLTENO® Single Plate (S1) Implant without interfering with the action of the extraocular muscles.
The MOLTENO® Double Plate Implants are suitable for patients with very severe glaucoma, patients with especially vigorous fibrous tissue response, younger patients with good ciliary body function and patients who have glaucoma associated with uveitis or retinal detachment (3). Most surgeons now prefer a Molteno3® Implant where they would have used a Molteno Double Plate Implant in the past (2).
This implant is available in left eye (L2) and right eye (R2) configurations. All MOLTENO® glaucoma implants are MRI safe.
Molteno® Pressure Ridge, Double Plate Implant (DL2 & DR2)
The Molteno® Pressure Ridge Double Plate Implant combines the large drainage area of the MOLTENO® Double Plate Implant with the subsidiary ridge for controlling postoperative hypotony. This implant is suitable for patients with very severe glaucoma, younger patients with good ciliary body function, patients with especially vigorous fibrous tissue response and patients who have glaucoma associated with uveitis or retinal detachment (3).
Most surgeons now prefer to use a Molteno3® implant where they would have used a MOLTENO® Double Plate Implant in the past (2).
The MOLTENO® Pressure Ridge Double Plate Implant is available in left eye (DL2) and right eye (DR2) configurations. All MOLTENO® glaucoma implants are MRI safe.
Microphthalmic Implant
MOLTENO® Paediatric / Microphthalmic Implant (P1)
The MOLTENO® Paediatric/ Microphthalmic Implant (P1) is a mini version (plate diameter 8mm) of the original MOLTENO® Single Plate (S1) Implant with a reduced radius of curvature suitable for microphthalmic eyes.
The MOLTENO® Paediatric /Microphthalmic Implant is indicated for eyes with an axial length of less than 17mm. The newer design Molteno3 Single Plate SS (185mm2) implant can be used for eyes with an axial length of 17mm or greater (a normal eye has an axial length of approximately 22mm).
In a young patient, positioning the MOLTENO® glaucoma implant in an anterior position, between the insertions of the extraocular muscles, reduces the impact of subsequent growth of the globe on the length of Molteno implant tubing in the anterior chamber (AC) and reduces the need for re-operation.
MRI safe.
Training
MOLTENO3 Presentation by Dr. Mark Latina For Glaucoma Surgery
MOLTENO3 Presentation by Dr. Mark Latina For Glaucoma Surgery
MOLTENO3 Implantation with Kerasys – By Dr. Robert Noecker
Get Product Quote
You may place your order now (PayPal invoice is available).
Fast and secure payment with 
Or Email Us To Place an Order!
info@brandife.in