AmnioTek™-P
About
Introducing the new and innovative AmnioTek™-P Processed Human Amniotic Membrane representing the latest in regenerative biologics technology for the ocular surface

Viable
AMT has been successfully employed in the surgical treatment of the ocular surface in well over 100,000 cases over the past decade
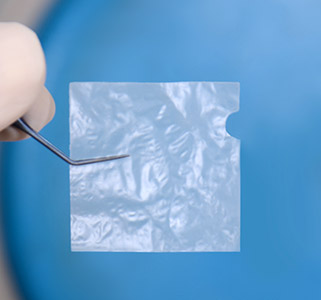
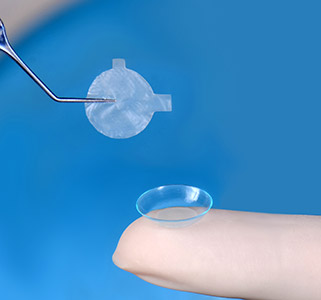
AmnioTek™-P offers the ophthalmic surgeon an effective, natural, minimally processed dehydrated, device-like amniotic membrane tissue featuring anti-inflammatory and anti-adhesive properties ideal for wound covering and wound healing applications.
AmnioTek™-P is a proven effective treatment for a host of ocular surface conditions.
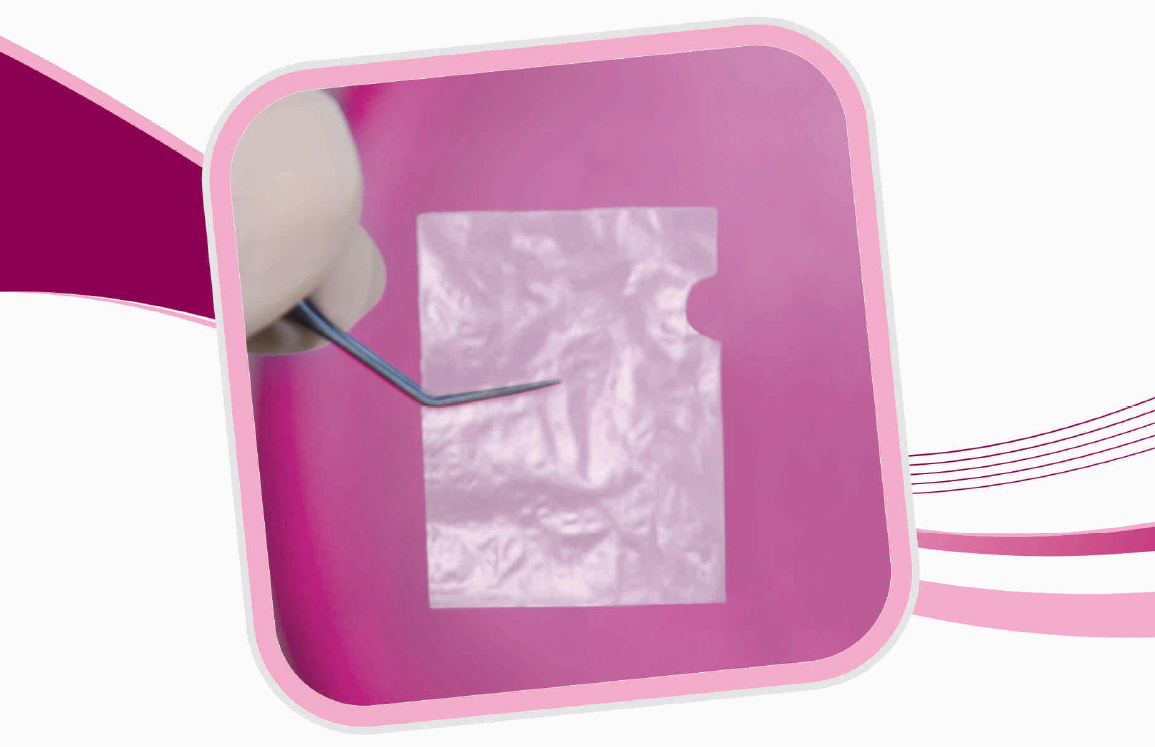
Packaging Cover

Step by Step
Step by Step process

Specification
Safety and sterility
AmnioTek™-P amniotic tissue meets the most stringent donor screening and serological testing standards to ensure safety. AmnioTek™-P is delivered sterile, dry and with a 3 year room-temperature shelf-life greatly reducing storage and shipping logistical costs incurred by other frozen allograft technologies.
Ophthalmic Indications
The main ophthalmic indications are:
- Pterygium surgery
- Ocular surface disease
- Corneal epithelial defects
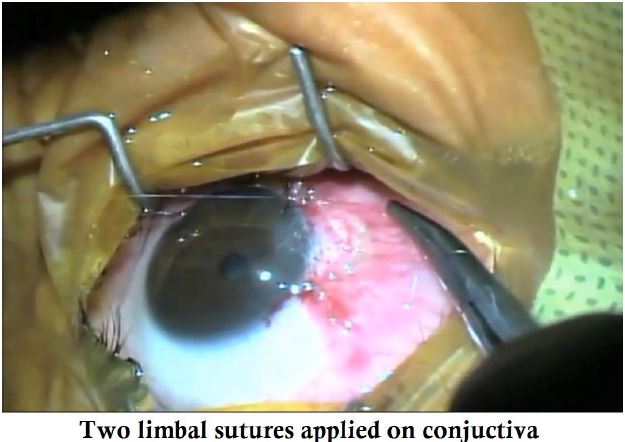
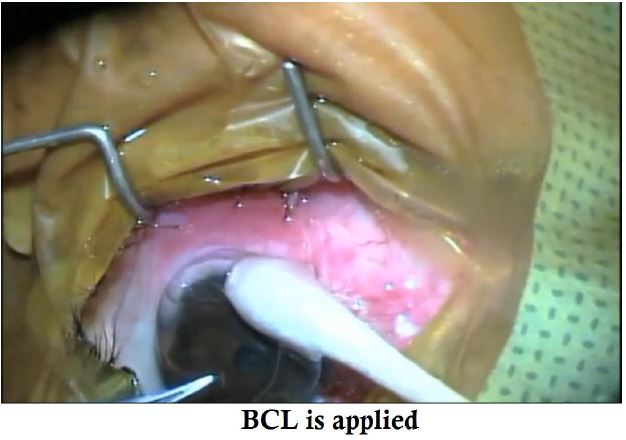
Instructions for surgery:
The main ophthalmic indications are:
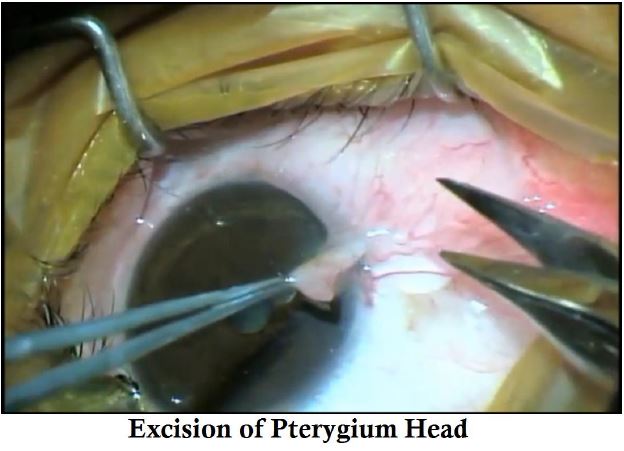
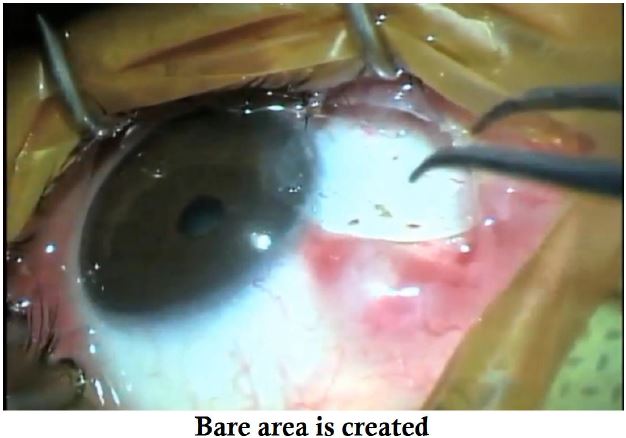
- The pterygium head is excised to create a pocket for the graft.
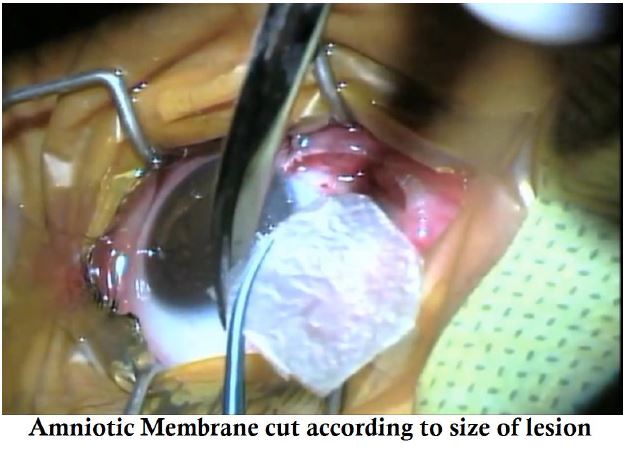
- AmnioTek-P is cut according to the size of the defect.
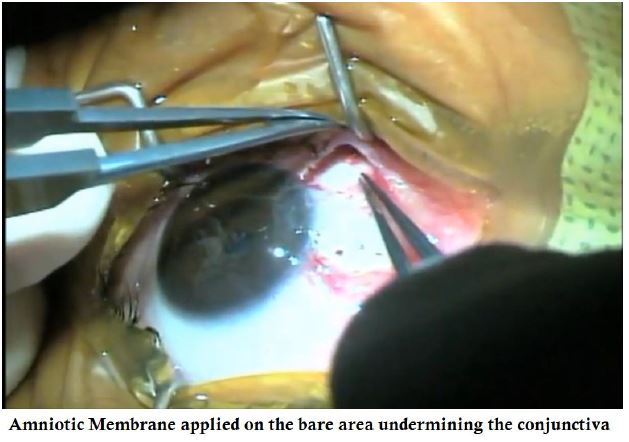
- AmnioTek-P is slid under the conjuctiva on the bare area.
- The conjuctiva is pulled up & sutured on the limbus and a BCL is placed.

Indication For Uses (IFU)


FAQ

Testimoninals

Get Product Quote